
Description
Use of purified water
non-aseptic drug ingredients, equipment, appliances and packaging materials in direct contact with the drug last washing water, non-aseptic raw material drug refining process water, water for injection preparation and pure steam water source.
Purified water quality requirements
|
Items |
Ch.P 2010 |
EP6.7 |
USP 32 |
|
Characteristics |
Clear and colorless liquid, odorless and tasteless |
Clear and colorless liquid |
Clear and colorless liquid |
|
pH |
Comply with the standard |
- |
- |
|
Chloride, sulfate and calcium salts |
- |
- |
- |
|
nitrate |
≤0.000006% |
≤0.2ppm |
- |
|
Nitrite |
≤0.000002% |
- |
- |
|
ammonia |
≤0.00003% |
- |
- |
|
CO2 |
- |
- |
- |
|
oxidizable substances |
TOC≤0.5mg/L |
TOC≤0.5mg/L |
TOC≤0.5mg/L |
|
nonvolatile matter |
≤1mg/100ml |
- |
- |
|
heavy metal |
≤0.00001% |
≤0.1 ppm (According to the Pharmacopoeia: if the conductivity meets the requirements of water for injection, this item can be omitted.) |
- |
|
electroconductibility |
Comply with the standard |
Comply with the standard |
Comply with the standard |
|
Microbial limit |
Total number of bacteria, molds and yeast:≤100/ml(Nutrient Agar, Rose Bengal Sodium Agar Medium) |
Total number of aerobic bacteria: ≤100/ml (The samples are cultured in AGAR medium S at 30 to 35 ° C for 5 days. |
Total number of bacterial colony≤100cfu/ml. (The samples are cultured in High nutrient medium, such as TGYA、m-HPC agar) at 30 to 35 ° C for 48~72 hours, or in low nutrient medium such as R2A Agar and HPCA at 20 to 25℃ for more time, such as 14 days.) Some tests to control bacteria can also be carried out |
Typical configuration of purified water preparation system:
Usually, the system configuration is different due to different regions and water sources. It is necessary to analyze and calculate the raw water quality, and then configure the corresponding components to purify water so that each index is within the allowable range.
At present, the main configuration of purified water preparation system is shown in the figure.
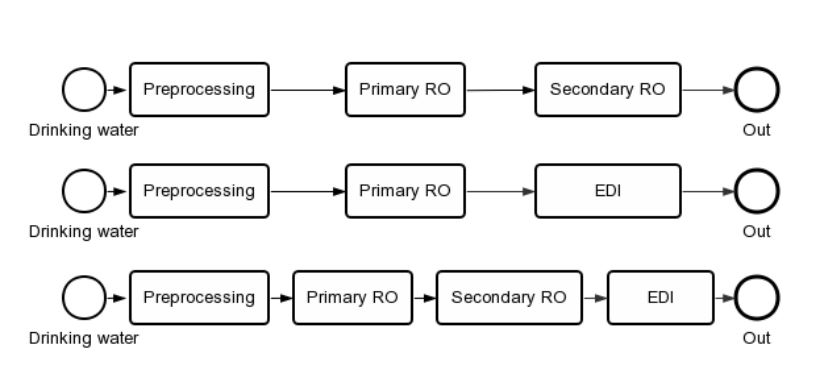
Design and application advantages of purified water preparation system
The equipment is mainly composed of pretreatment system, filter, pH dosing device, secondary reverse osmosis system, EDI system and electrical control system.
- Double water inlet circuit design can effectively control the growth of microorganisms.
- Rinsing RO membrane with fresh water can prolong its service life.
- Preparation of purified water does not require intermediate storage tanks, which can effectively control the growth of microorganisms and save investment costs.
- The system adopts intermittent mode to run stably and save energy.
Previous
Next
Previous
Next








